The Coalition for Epidemic Preparedness Innovations (CEPI) is conducting clinical research studies to develop a vaccine for Lassa fever, a deadly hemorrhagic fever prevalent in West Africa.
The ENABLE 1.5 study, launched in collaboration with the governments of Nigeria, Liberia, and Sierra Leone, builds on the progress made from ENABLE 1.0, which ran from 2020 to 2024 as the largest-ever epidemiological study on Lassa fever.
ENABLE 1.5 will deepen scientists’ understanding of the transmission, epidemiology, and impact of Lassa fever in Nigeria and across West Africa, focusing on generating evidence for public health strategies and advancing vaccine development.
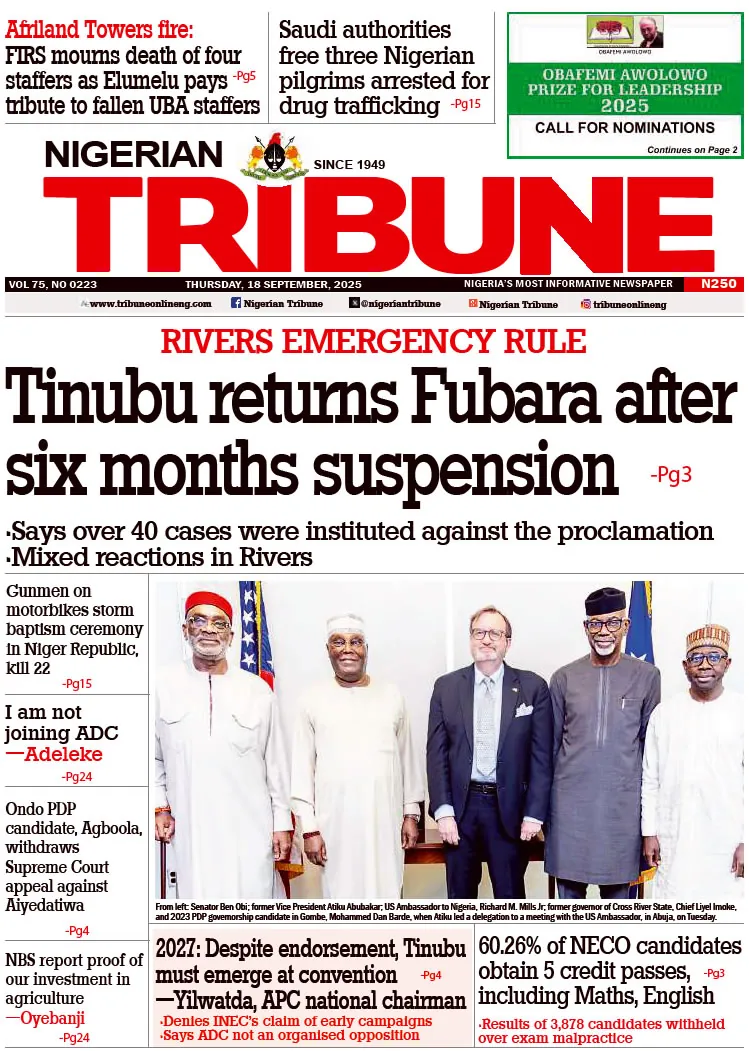
The National Project Coordinator for ENABLE 1.5 in Nigeria, Dr. Elsie Ilori, emphasized at the Study Kick-Off Meeting in Abuja that the main purpose of this study is to produce a vaccine to reduce the burden of Lassa fever in Nigeria and West Africa.
“The study will help us understand Lassa fever itself, how people react to the disease, and the effects of the vaccine on individuals. By understanding the disease, we’ll be able to understand how the vaccine will work in people,” she stated.
ALSO READ: Independence Day: Tinubu to make national broadcast Tuesday
“The study aims to understand Lassa fever’s symptoms and effects on people, evaluate the vaccine’s safety, tolerability, and immunogenicity, and develop a vaccine effective against multiple strains of the disease.”
Over 600 participants from Nigeria, Liberia, and Ghana will be enrolled. Selection criteria include age, with adults, adolescents, and children as young as two years old eligible, as well as individuals living with HIV. Participants will be selected from sites with a high Lassa fever burden, such as FMC Owo, ISTH Irrua, and AE Futa in Abakaliki, Nigeria.
The study prioritizes community engagement and sensitization. Researchers will conduct house-to-house enrollment, obtaining consent from household heads. Participants must provide informed consent and can withdraw at any time without consequences.
“We’re working closely with community leaders, healthcare workers, and local authorities to sensitize the public about the study,” Dr. Ilori said. “This includes explaining the study’s objectives, benefits, and potential risks.”
“These sites were selected due to their high disease burden, increasing the likelihood of enrolling participants with Lassa fever,” Dr. Ilori noted.
Dr. Ilori emphasized that “this study will help sensitize people and prepare them for vaccine acceptance.” With Lassa fever’s devastating impact on West Africa, this vaccine offers hope for reducing the disease’s burden.
“The study’s success relies on collaboration between researchers, healthcare workers, and community leaders from Nigeria, Sierra Leone, and Liberia,” Dr. Ilori concluded. “We believe that this study will pave the way for a vaccine, bringing relief to communities ravaged by the disease.”
ALSO READ: Independence Day: Tinubu to make national broadcast Tuesday
The study’s findings will also inform public health policy and guide future vaccine development. “We’re committed to ensuring that the vaccine is accessible and affordable for those who need it most,” Dr. Ilori added.
In addition to CEPI, the study is supported by global health partners, including the World Health Organization and the Nigerian government. “We’re grateful for their support and expertise,” Dr. Ilori said.
As the study progresses, researchers will closely monitor participants’ safety and vaccine efficacy. “We’re optimistic about the potential of this vaccine to save lives and protect communities,” Dr. Ilori said.
The study’s impact extends beyond West Africa. “Lassa fever is a global health concern, and a vaccine would benefit people worldwide,” Dr. Ilori noted.
With the study underway, hope is on the horizon for those affected by Lassa fever. “We’re one step closer to a vaccine, and that’s a remarkable achievement,” she added.
Similarly, the Consultant Public Health Physician and Data Manager for ENABLE 1.5 at the Irrua Specialist Teaching Hospital in Edo State explained that the hospital’s expertise in handling Lassa fever cases is evident, with referrals coming in from various clinics.
He added that the hospital is well-equipped with staff and equipment to manage Lassa fever cases; however, he noted that the burden remains high, with many cases reported globally.
He stated that the Coalition for Epidemic Preparedness and Innovation (CEPI) initiated an epidemiological study to develop a vaccine, leading to the improvement of ENABLE 1.0 and the upcoming ENABLE 1.5.
“The vaccine development process is underway, with Phase 1 and Phase 2A already ongoing. Phase 2B will commence soon across various sites.”
“This vaccine aims to reduce the severity and minimize symptoms of Lassa fever, ultimately decreasing its burden. The post-pandemic study focuses on understanding Lassa fever’s symptoms, effects, and vaccine efficacy.”
“As a participating site, the Irrua Specialist Teaching Hospital will contribute to the study’s objectives.”
“The study aims to evaluate vaccine safety, tolerability, and immunogenicity, understand Lassa fever’s symptoms and effects, and develop a vaccine effective against multiple strains.”
“With the vaccine expected to be available within the next year, the hospital’s involvement will play a crucial role in reducing Lassa fever’s burden and improving public health outcomes,” he said.
The Consultant Public Health Physician at FMC Owo in Ondo State, Dr. Ayodeji Oladele Olufemi, disclosed that Nigeria’s journey to developing a Lassa fever vaccine is underway, with Phase 2A trials currently ongoing in Abuja and Ghana.
He stated that the goal is to complete this phase by early next year, paving the way for Phase 2B trials across four sites in Nigeria, which will take about a year.
“After that, the Phase 3 clinical trial will begin, and this is expected to take at least one to two years. So, in total, we’re looking at approximately three years before the vaccine is ready for public use.”
“It’s exciting to see the progress being made, especially since Lassa fever claims around 5,000 lives annually in West Africa. The vaccine candidate has shown promising results in previous trials, with robust immune responses sustained for up to one year after vaccination.”
“The Coalition for Epidemic Preparedness and Innovation (CEPI) is funding this research, with the aim of making the vaccine accessible and affordable for all populations in need.”
“The Phase 2B trials are expected to start early next year across four sites in Nigeria. The Phase 3 clinical trial will begin immediately after the completion of Phase 2B and is expected to take around one to two years. Consequently, the vaccine is expected to be available in approximately three years.”
Meanwhile, CEPI Programme Manager Roice Fulton explained that ever since we identified Lassa fever as one of our priority pathogens, CEPI has been working with the World Health Organization’s Blueprint for Lassa and other research priorities to identify new therapeutics and vaccines for these neglected tropical diseases.
He stated, “We realized that it’s not just important to try and develop vaccines through clinical trials but to do the additional groundwork necessary to identify where the disease is, what sorts of individuals have the disease, and where we can find locations to recruit new subjects for these clinical trials.”
“But on top of that, we need to build the necessary capacities within the countries and our partners in Nigeria and other countries to prepare for the significant work ahead, which is to conduct large-scale clinical trials that will allow us to identify how effective a new vaccine will be, with the ambition of having that vaccine ready by 2030 here in Nigeria.”
“We believe that there is much yet to be done for preparedness and readiness to ensure that the sites and countries are genuinely equipped with the knowledge, expertise, and tools to conduct large-scale clinical trials,” he added.
WATCH TOP VIDEOS FROM NIGERIAN TRIBUNE TV
- Relationship Hangout: Public vs Private Proposals – Which Truly Wins in Love?
- “No” Is a Complete Sentence: Why You Should Stop Feeling Guilty
- Relationship Hangout: Friendship Talk 2025 – How to Be a Good Friend & Big Questions on Friendship
- Police Overpower Armed Robbers in Ibadan After Fierce Struggle